Past Research
Natural Gas Transportation and Storage
Natural gas is predicted to see an increase in usage due to its lower carbon dioxide emissions compared to coal and oil. However, one drawback is that, in its standard state as a gas, it does not have a high energy density per volume.
Japan imports natural gas in the form of Liquefied Natural Gas (LNG). While LNG has a density 600 times greater than its gaseous form, the facilities required for its production, transportation, and storage must be made of materials that can withstand its extremely low temperature.
An alternative method for transporting and storing natural gas is through Natural Gas Hydrates (NGH). Although the density of NGH is only about 160 times greater than that of gaseous natural gas, it can be stored at relatively higher temperatures. We have been performing research to measure the formation and decomposition conditions of hydrates under the conditions required for storing NGH.
Alcohol Hydrates
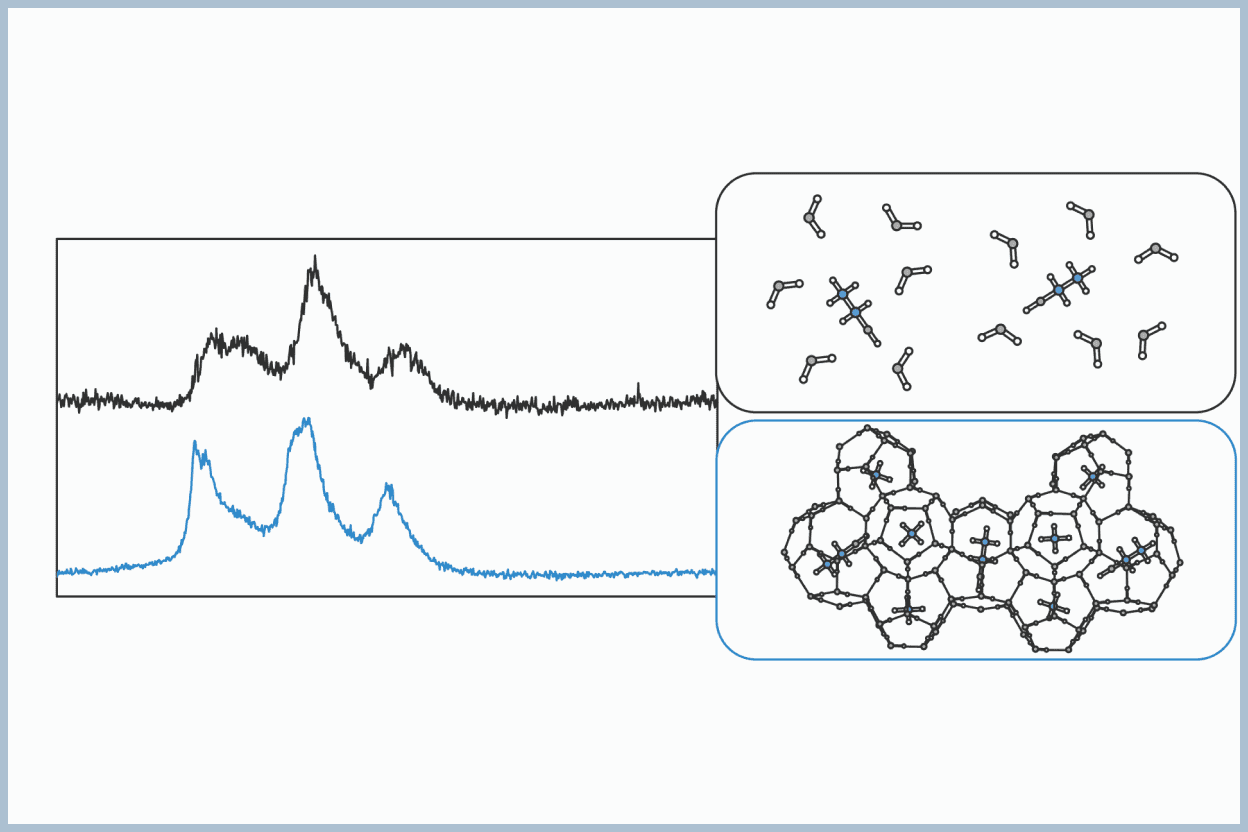
Generally, the guest compounds in hydrates are hydrophobic, meaning they do not dissolve well in water. This is because the inclusion of hydrophobic guest molecules in the hydrate cages stabilizes the crystal structure. For many years, it has been believed that hydrophilic compounds, such as alcohols, which dissolve well in water, do not become guests in hydrates. In particular, small alcohols like ethanol and propanol have been considered inhibitors of hydrate formation.
We have found that ethanol and propanol can become guests in hydrates along with methane. This finding not only impacts the use of alcohols as hydrate formation inhibitors but also presents an interesting phenomenon from a physicochemical perspective, as it raises the question of how water molecules that make up the hydrate cages interact with hydrophilic substances when they become guests.

Air Hydrates in Ice Sheets
Understanding past climate changes on Earth is crucial when discussing global warming. Since humans began performing meteorological observations in their current form around the 19th century, it is necessary to reconstruct climate changes prior to that time from traces left on the planet. One such method involves using ice sheets found in Antarctica and Greenland. In these regions, snow that accumulates during winter does not melt in the summer, and each year's snowfall forms layers of ice. This creates ice sheets, with an average thickness of 2,500 meters in Antarctica. When snow is compressed into ice, it traps the surrounding atmosphere. As a result, ancient air is enclosed within the ice sheets, and analyzing it can reveal the climate of that time. This analysis has uncovered climate data going back about 700,000 years. Components of the air, such as nitrogen, oxygen, argon, and carbon dioxide, can all become guests in hydrates. Therefore, air hydrates are formed in the deep parts of the ice sheets as the trapped air reacts with the surrounding ice. This affects the analysis of past climate changes.
We have been performing research to measure the conditions under which air hydrates form and to investigate the correspondence between the actual distribution of air hydrates in ice sheets and the measured conditions.
Refrigerant for Air Conditioning and Refrigeration Systems
Hydrate formation is an exothermic reaction that releases heat to the surroundings, while hydrate decomposition is an endothermic reaction that absorbs heat from the surroundings. These reactions are being considered for use in air conditioning systems and refrigeration units by employing hydrates as working media. Since the conditions for hydrate formation and decomposition vary depending on the guest compounds, it is possible to create hydrates that form and decompose under suitable conditions for air conditioning and refrigeration systems by selecting the appropriate guest.
So far, we have performed research on measuring the formation and decomposition conditions for finding suitable guests, as well as on hydrates with alcohol guests suitable for refrigeration units operating at temperatures lower than those of conventional freezers.

Mist Jet
Mist jet refers to the air jet impingement coexisting with sprayed water droplets. It is an efficient cooling technology that cools an object by impingement with high flow velocity. Heat is invisible, but it is essential to remove or add heat to maintain the appropriate temperature of any technology. The mist jet takes advantage of the property that water absorbs heat from the surroundings as it evaporates. Although it is a simple mechanism of adding water to the air, it is known to have excellent cooling effects.
In our group, we have investigated how much cooling effect can be achieved by spraying only a small amount of mist without wetting the object. By doing so, we aim to use it for cooling electronic devices or hybrid car batteries.
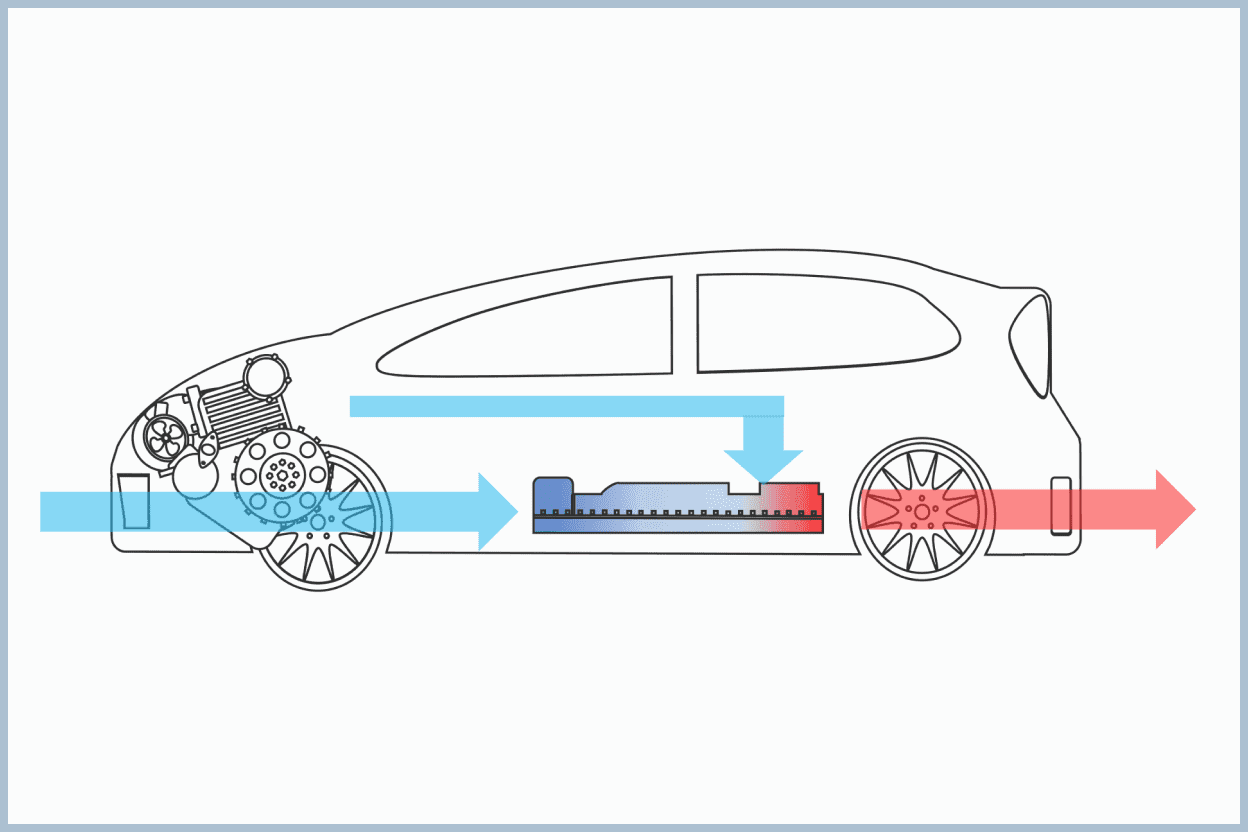
Cooling of Hybrid Vehicle Batteries
To reduce the emission of carbon dioxide, a greenhouse gas, into the atmosphere in the transportation sector, the promotion of electric cars and hybrid cars, equipped with batteries and motors in addition to engines, is being advanced. To operate these cars, which use batteries for energy storage instead of gasoline, efficiently and safely, battery temperature management is crucial. Temperature management can prevent thermal runaway and increase energy efficiency, especially in hot weather.
We have performed research on the heat transfer characteristics of a mist jet cooling mechanism for batteries during the operation of the engine in a hybrid car. This mechanism can effectively cool the battery without increasing costs by using a combination of existing components.