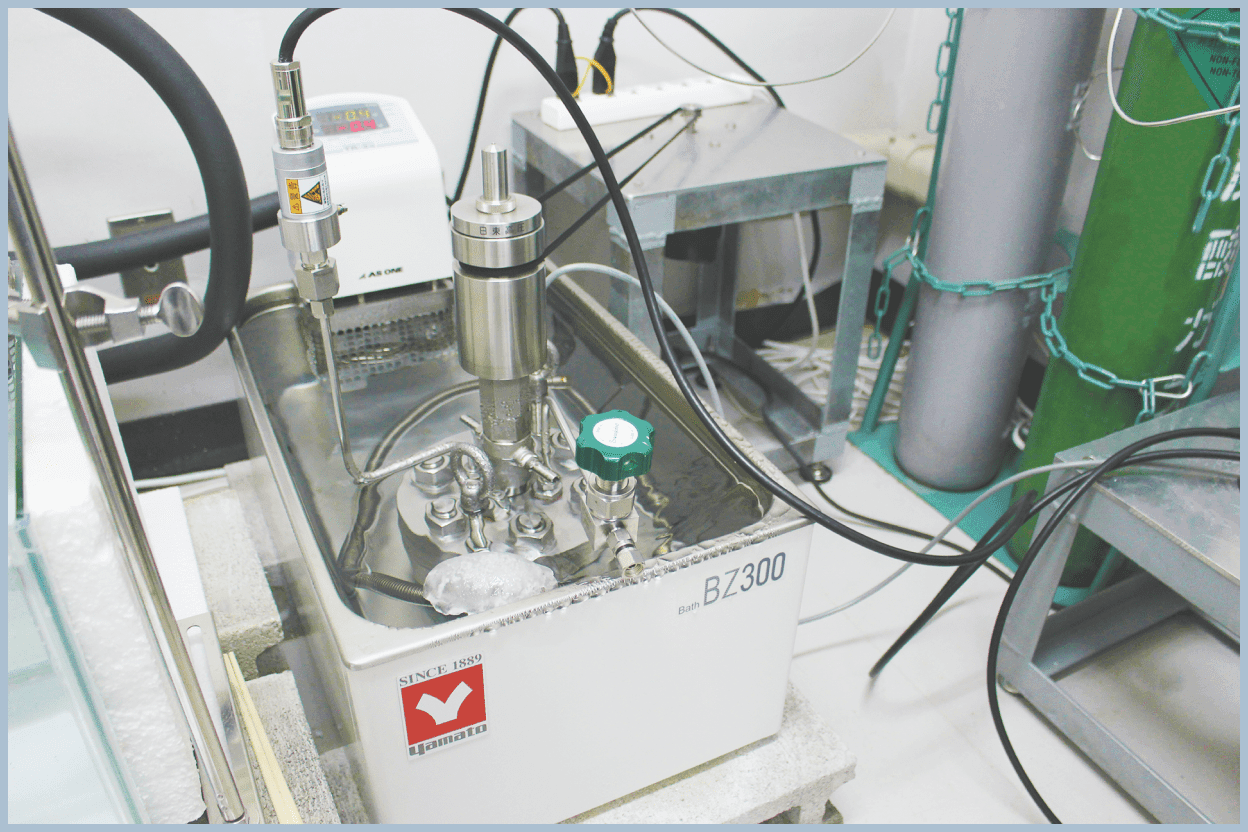
Phase Equilibrium Conditions Measurements
Phase equilibrium conditions correspond to the conditions under which hydrates form and decompose. They are fundamental data for all hydrate-related technologies. Phase equilibrium conditions vary depending on factors such as the type of guest compounds and the salinity of seawater, so it is necessary to measure phase equilibrium conditions that are suitable for each technology.
In our group, we perform experiments to form and decompose hydrates inside pressure vessels capable of withstanding high pressures, and allow them to reach equilibrium over time. By measuring temperature and pressure, we determine the equilibrium conditions.
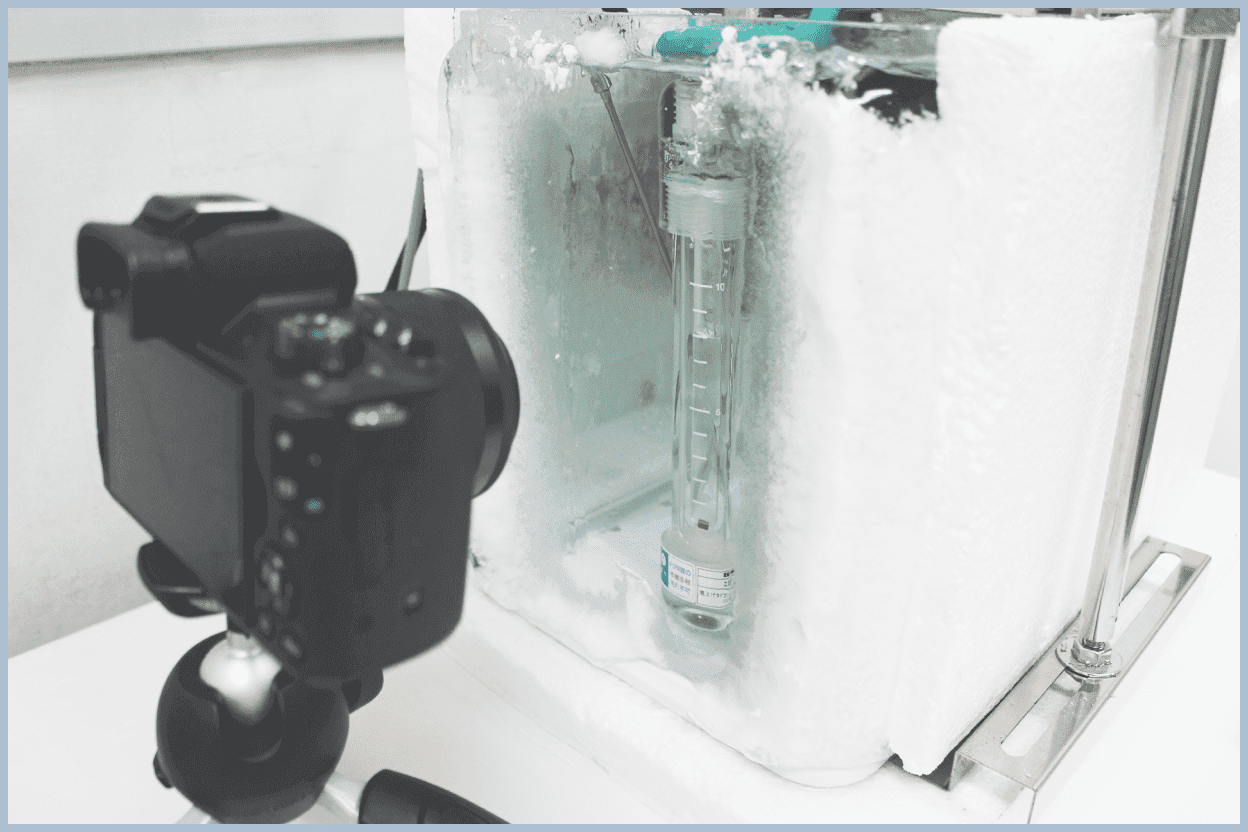
Crystal Growth
Hydrates are solid crystals. When utilizing hydrates in various technologies, understanding how hydrates form inside a reaction vessel and how they interact with the surrounding water and guest phases is effective for promoting hydrate formation reactions and designing reaction vessels.
We perform experiments to form hydrates in an observable experimental system using glass pressure vessels and glassware commonly used in chemical experiments, and observe the formed crystals.
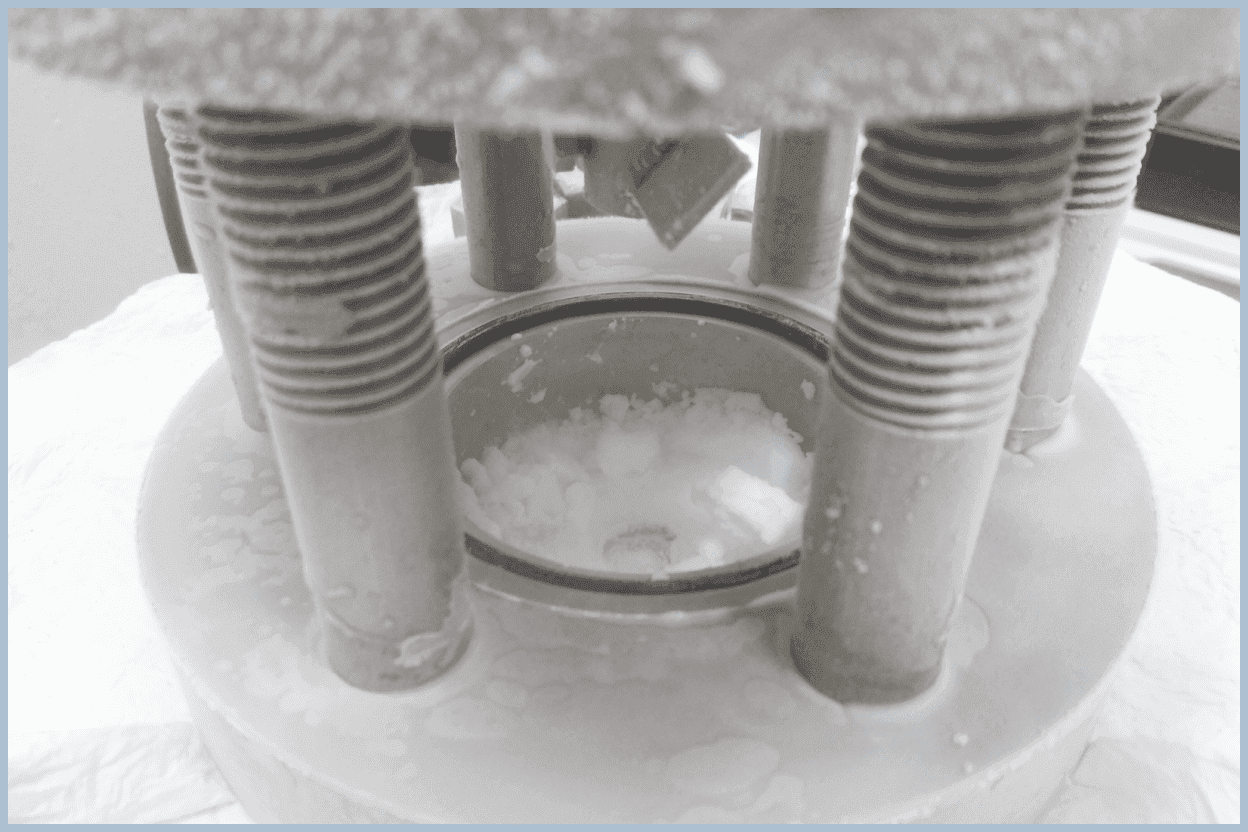
Sample Preparation
Crystals are solids formed by molecules or atoms arranged in a regular pattern. Since we cannot observe molecular or atomic movements with our eyes, special analyses are useful for revealing the structure of crystals. To perform such analyses, it is necessary to form high-purity hydrate samples.
We form high-purity samples using pressure vessels and collaborate with other institutions to analyze them at the molecular and atomic levels. Hydrates formed in the same way can also be viewed during open campus events or laboratory tours.
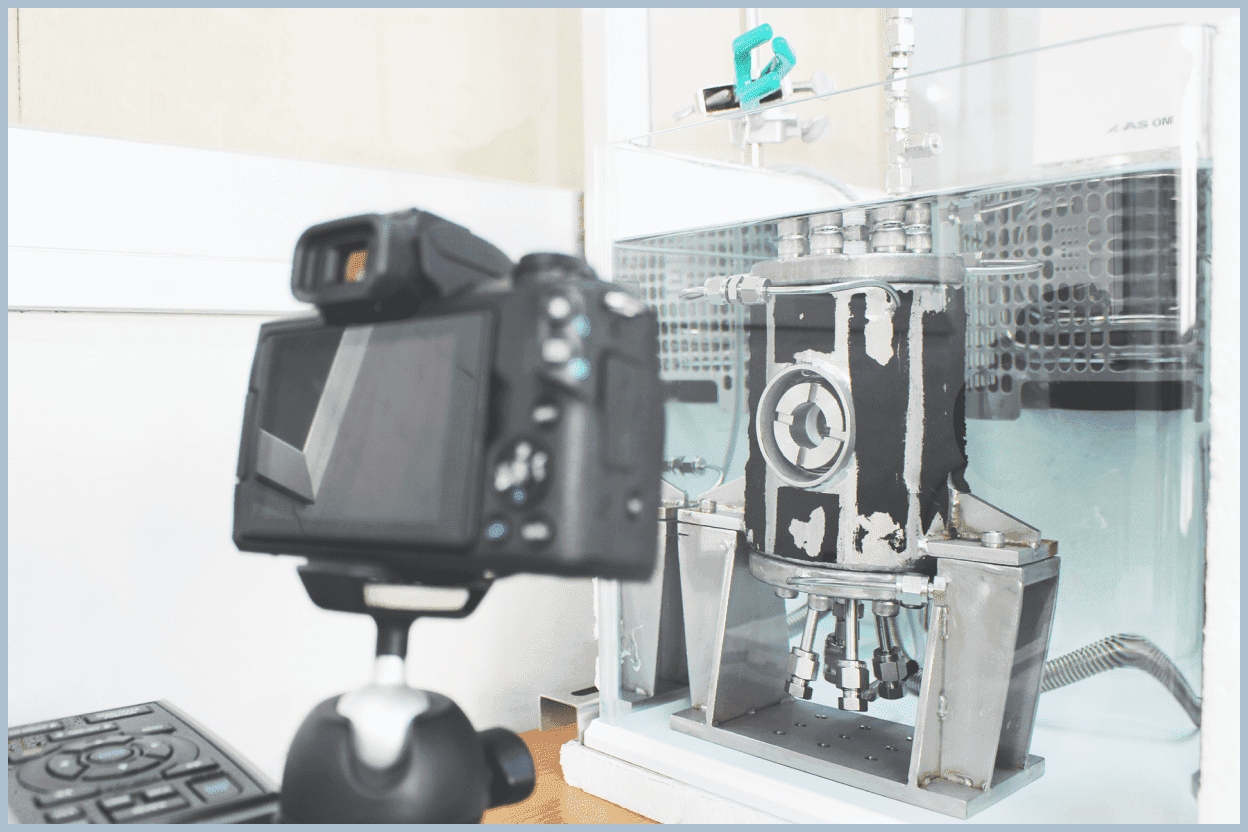
Interfacial Tension Measurements
Hydrate formation occurs at the interface where water and guest phases meet, specifically at the gas-liquid interface. To promote formation, methods such as creating guest gas bubbles in liquid water or spraying water as a mist into the guest gas are often employed. The shape of bubbles and droplets is determined by surface tension, or more precisely, interfacial tension.
We perform experiments to measure the interfacial tension between water and guest phases at the gas-liquid interface under conditions close to hydrate formation conditions. The experiments involve forming droplets in the guest gas inside an observable pressure vessel and analyzing images taken of the droplets.
Ion Chromatograph and Gas Chromatograph
Seawater contains various dissolved components as salts. Therefore, in order to establish seawater desalination/salt production technology, it is important to know how the concentrations of each component in seawater change due to hydrate formation. On the other hand, for carbon dioxide separation technology, natural gas transport and storage technology, and air hydrate analysis, it is essential to perform gas composition analysis since the target guest is a mixed gas.
In our group, we use ion chromatograph and gas chromatograph owned by Center for Research Advancement and Collaboration at the University of the Ryukyus to measure the composition of liquids and gases.

