Clathrate Hydrates
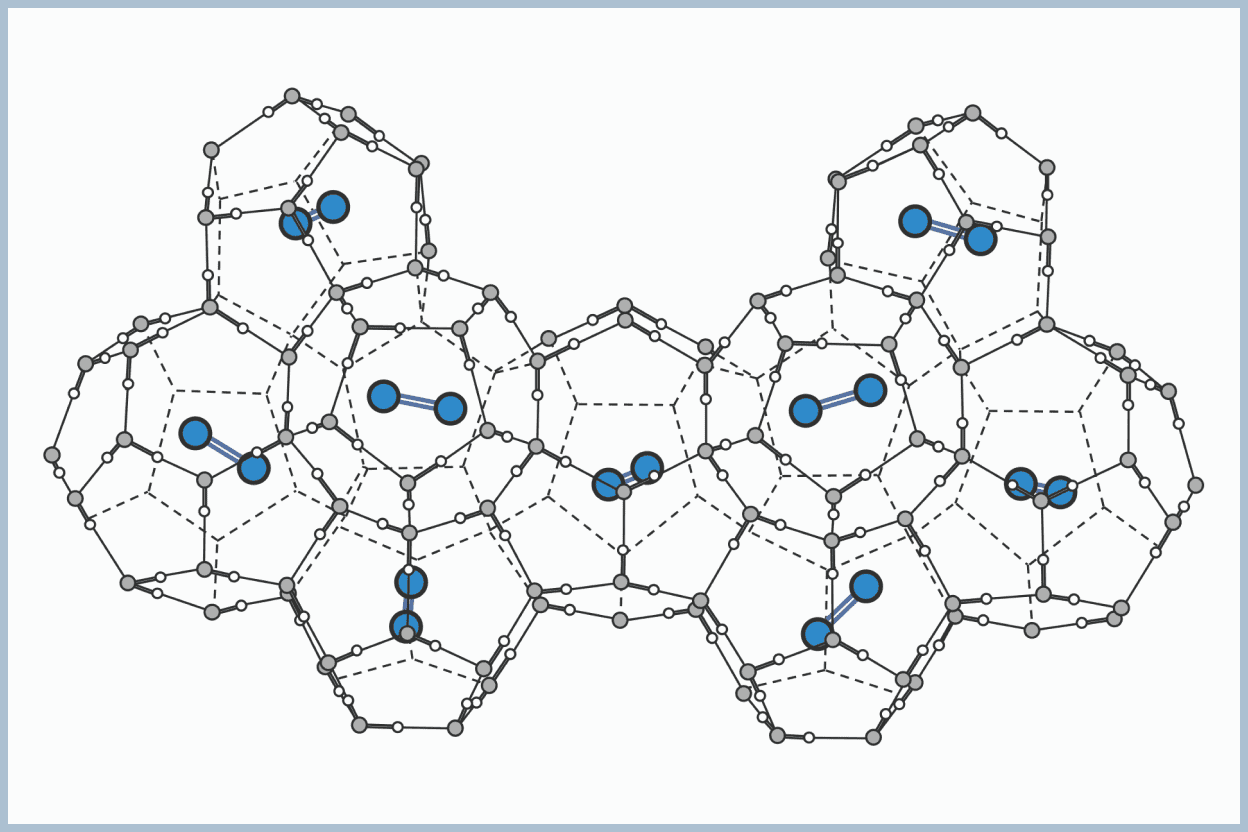
Clathrate hydrates (hereinafter referred to as hydrates) are ice-like solid compounds formed by water molecules creating a cage-like structure through hydrogen bonding, and being occupied by "guest" molecules within.
There are various guests, ranging from hydrocarbons such as methane and ethane, to rare gases such as xenon and argon, to nitrogen, oxygen, carbon dioxide, and other components that constitute the atmosphere. The physical properties of hydrates vary depending on the guest.
In hydrate-related research, it is important to measure the properties and to clarify the phenomena of their formation and decomposition. It contributes to achieving the SDGs, such as carbon dioxide separation and recovery, and can even relate to the formation of planets and satellites. Hydrates are a mysterious substance produced by the water that is close to us.
The History of Clathrate Hydrates and Our Research
The first artificial clathrate hydrate was created just over 200 years ago in 1810. In the 1930s, it was discovered to be a cause of blockages in oil and natural gas pipelines. In the 1960s, it was found that large quantities of methane hydrates exist in deep-sea floors and permafrost regions around the world, including those around Japan. Development of clathrate hydrates as an energy resource has continued since then. Based on the physical properties of clathrate hydrates revealed in these studies, attempts have been made to actively form and decompose them for use in industrial technologies.
Currently, research and development on clathrate hydrates are focused on three pillars: preventing pipeline blockages, developing methane hydrate resources, and clathrate-hydrate-based technologies.
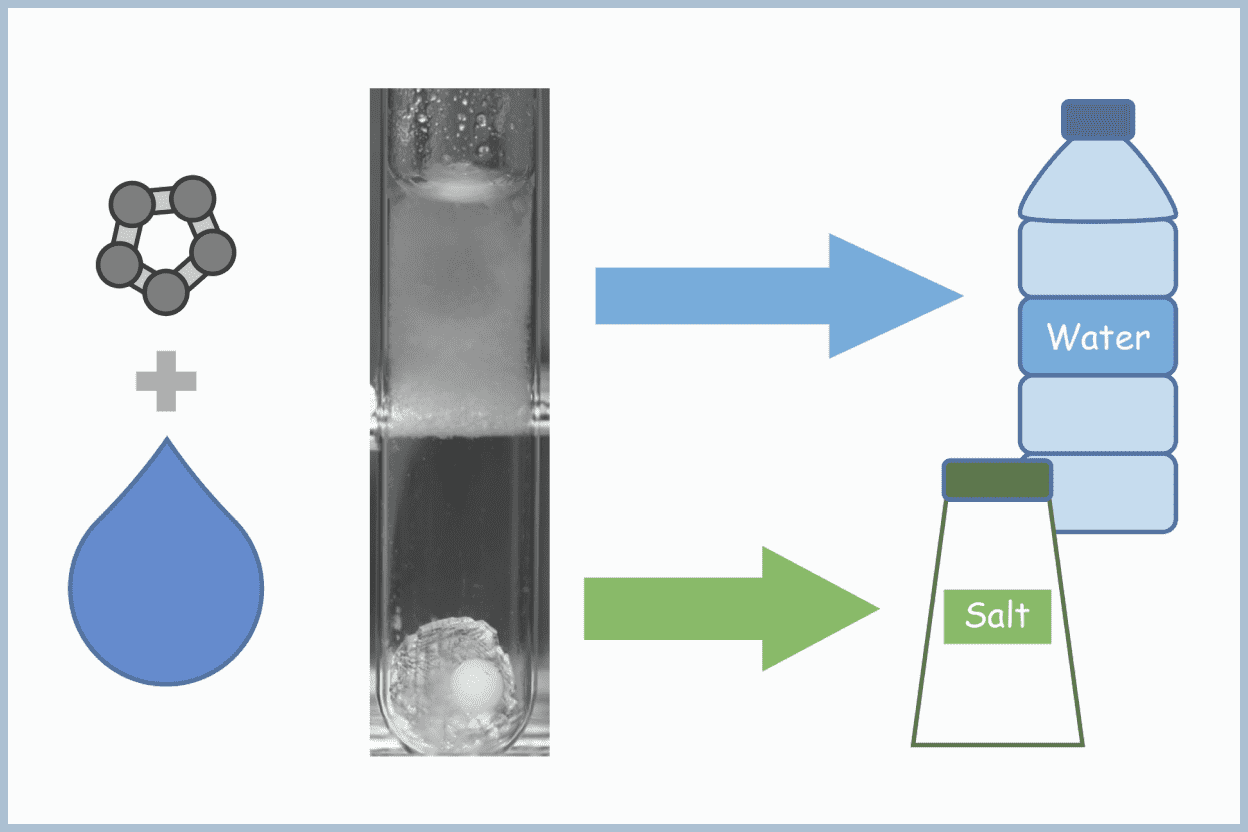
In our group, we are focusing on the development of technologies that actively utilize clathrate hydrates. Recently, we have been performing research on seawater desalination/salt production technology, lithium concentration technology, and carbon dioxide capture and sequestration technology.

